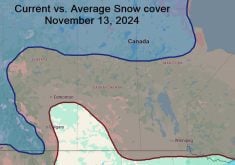
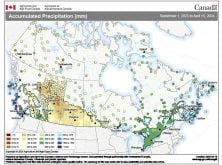
Sometimes you just have to seize a teachable moment and take a break from the normal routine of a class, and this week looks to be just that week. While I’m not sure just what will happen with the storm system that is to move through southern and central Manitoba the first two to four days of this week, it looks like we will see a mixed bag of precipitation – anything from rain, freezing rain and sleet to snow. So I figured we should take a look at these four different forms of precipitation and explore the type of atmospheric conditions that lead to each of them.
Read Also

June brings drought relief to western Prairies
Farmers on the Canadian Prairies saw more rain in June than they did earlier in the 2025 growing season
To understand why different types of precipitation occur, we need to understand that temperatures can vary greatly as we go up and down in altitude. So far in our weather school discussions we have come to an understanding that cold air is heavy and dense, so it tends to sink and stay close to the ground. Warm air is light, and therefore tends to rise. We have also looked at the composition of the atmosphere and when we look at an “average atmosphere” we would expect temperatures to decrease as we move upward.
We now have to skip a couple of lessons and combine these ideas to help explain the different types of precipitation that can occur during the cold months of the year.
A snowflake’s journey
The first and most easily understood kind of winter precipitation is snow. In our part of the world the vast majority of moisture condensing in clouds is doing so in the form of snow. What determines whether that snow will make it to the ground is the temperature of the air that the snowflake has to fall through. If the temperature stays below the freezing point from the cloud to the ground, then we see it fall as snow.
If the temperature on the ground is below zero, but we have a push of warm air that has lifted over the cold, dense air at the surface, then the falling snow can start to melt on its way down to the surface. If the layer of warm air is not very thick, then the snowflake only partially melts before it reaches the subfreezing layer near the surface. Once it reaches this subfreezing layer, the partially melted snowflake then refreezes, turning into an ice or snow pellet. It takes this shape because the outer edges of the snowflake melt in the warm layer, creating a layer of water around the inner core of the snowflake. When the melted snowflake passes through the subf reezing surface layer, this coating of water freezes around the remaining snowflake and creates a hard shell around the soft inner core.
If we take the same scenario as above, but increase the thickness of the warm layer, our falling snowflake will totally melt and become a raindrop. This raindrop will then fall through a layer of subfreezing temperatures. Two things can happen at this point. If the air is cold enough, the raindrop will freeze solid and we will experience ice pellets, but this is fairly rare. What we will usually experience is that the water droplet will cool below the freezing point, but remain in a liquid state (we will discuss why this happens in a later lesson). Once this super-cooled water droplet or raindrop hits an already frozen surface, it will spread out and then immediately freeze, creating what we call freezing rain.
Finally, if the whole layer of the atmosphere between the formation of the snowflake and the surface of the Earth is above freezing, then there is a good chance that the snowflake will melt and remain in a liquid state until it reaches the Earth in the form of a raindrop.
Evaporative cooling
But– oh, you knew there was going to be a “but” – it is never just that simple. Just like when you study humans or animals, the objects you are studying can change the conditions on which the study is based. In this case, the falling snowflakes, ice pellets or raindrops can change or influence the temperature of the air around them, thus changing the conditions.
One thing that can happen is evaporative cooling, which can give us snow when the temperatures in the atmosphere would normally result in rain. If the atmosphere through which the snow falls is not totally saturated – that is, the relative humidity is not at 100 per cent – then the falling snow will start to evaporate as it begins to melt. This evaporative process requires energy, or heat, and this heat is taken from the surrounding atmosphere. This means the surrounding atmosphere begins to cool and eventually it may cool to the point where the temperature is no longer above freezing. Our snowflake will now no longer melt and instead of seeing freezing rain or ice pellets, all we see is snow.
So now you know the difference between all the different types of winter precipitation. Now I wonder just which types we will see from this week’s storm.