U.S. health regulators said they would work with their counterparts worldwide to share information and better safeguard drugs and food consumed in the United States.
The move represents a change in strategy for the U.S. Food and Drug Administration, a recognition of an increasingly complex global supply chain and tight budgets at home.
“The border can no longer be the nation’s primary line of defense against unsafe imported products,” FDA Commissioner Margaret Hamburg told reporters June 20.
About 45 per cent of fresh fruits and vegetables and 70 to 80 per cent of all seafood consumed in the United States are imported, the FDA said in a report.
Read Also
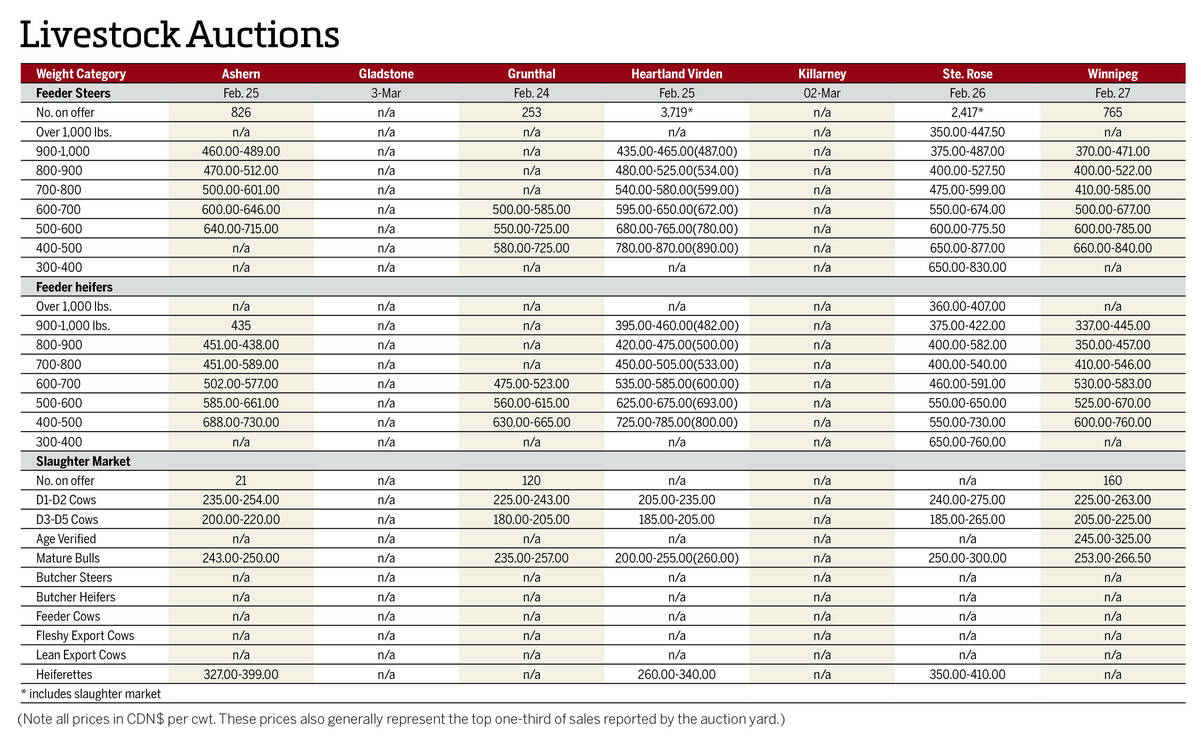
Manitoba cattle prices, March 4
Chart of weekly Manitoba cattle prices.
More drugs and medical devices are also made abroad, with some 80 per cent of the active ingredients in U.S. drugs manufactured overseas.
In the past, the FDA primarily relied on its own foreign inspections and border controls to ensure U.S. consumers got safe food and drugs.
But imports of FDA-regulated products are slated to triple between 2007 and 2015, while the FDA budget could be squeezed by fiscal austerity measures in Congress.
“Instead, the border must serve as a final checkpoint on preventive controls throughout the supply chain,” Hamburg said.














